Specialized microbes from the genus Geobacter generate energy by transferring electrons outside their cell membranes. In the environment, this alters metal solubility and toxicity. In anaerobic digestion this can divert electrons to methane production. In bioelectrochemical systems such as microbial fuel cells, Geobacter acts as the key engine converting waste into electricity.
Geobacter is like a power source that comes with many adapters. It tunes its electron transfer machinery in response to challenges posed by different environments.
Molecular basis of extracellular respiration
Many of our recent projects share a common thread: that Geobacter can sense different metals, surfaces, or environments outside the cell, and use different cytochromes to link its metabolism to their reduction.
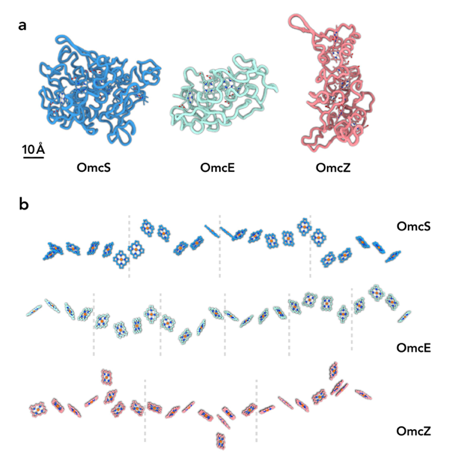
Multiple nanowire families: In collaboration with Fengbin Wang, Allon Hochbaum, and Ed Egelman, we have found two unique families of conductive cytochrome-based filaments. Filaments comprised of OmcE or OmcS, which are associated with metal reduction, insulate their hemes along the center of the protein, and contain modifications on their surface. While OmcE and OmcS share no sequence homology or similarity in their protein fold, their hemes can be aligned in the polymerized structure.
Filaments made of OmcZ, which are needed to grow as conductive biofilms, have a branched structure, with solvent-exposed hemes. The heme arrangement in OmcZ is completely unique from OmcE or OmcS, suggesting nanowires have evolved multiple times.
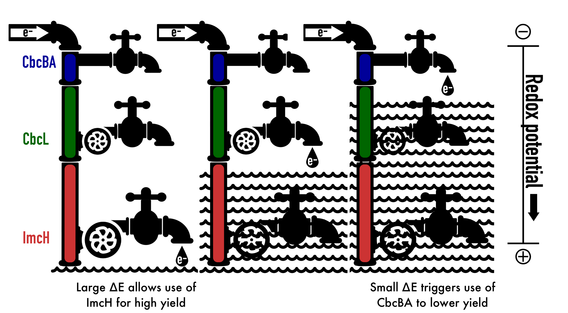
Electron transfer is potential-dependent: By controlling the inner membrane cytochrome required for growth with high-potential acceptors (Levar et al, ImcH), moderate-potential acceptors (Zacharoff et al, CbcL), or respiration near the thermodynamic limit (Joshi et al, CbcBA) electron transfer to electrodes or metals can be controlled and be gated to only operate at specific voltages (Chan et al, 2015). This allows cells to maximize growth yields as the environment changes. Recent work tracing the voltage-dependence of one of these proteins to specific amino acid residues provides an explanation for how Geobacter can so rapidly adapt to changing conditions.
Surface sensing: Geobacter sulfurreducens has a chemosensory gene cluster essential for growth using an electrode, but dispensable for growth using metals. Deletions of esn ('electrode sensing network') components—such as the methyl-accepting chemotaxis protein (MCP) (esnA), a CheW-like scaffolding protein (esnB), and a CheA-like histidine kinase (esnC)—severely diminishes electrode growth. However, deletion of these genes does not affect reduction of metals.
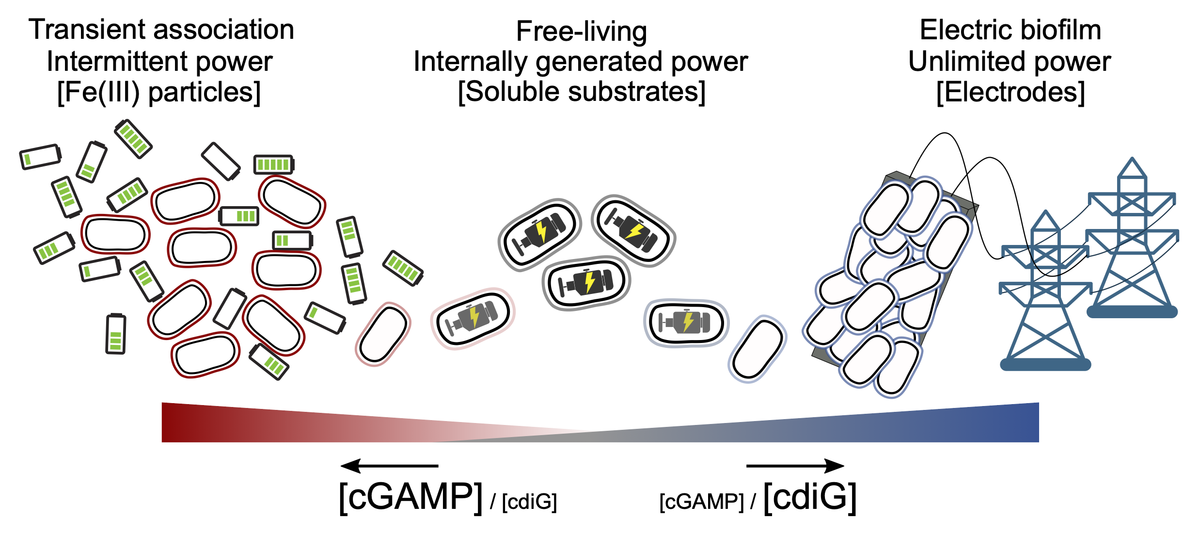
New second messengers involved in sensing: Our collaboration with the Hammond Lab describes how the hybrid dinucleotide cyclic AMP-GMP coordinates cytochrome expression and attachment mechanisms. A unique GGDEF-family cyclase (GacA) controlled by esn sensing produces the cyclic AMP-GMP, which activates riboswitches upstream of key cytochrome and pilus assembly operons, but only for metal reduction. Growth on electrodes does not require cyclic AMP-GMP, but instead is linked to standard cyclic di-GMP signaling driven by esn genes. This further underscores how Geobacter can tell the difference between difference extracellular acceptors.
New outer membrane cytochrome conduits: Porin-cytochrome electron conduits consist of an integral β-barrel through which periplasmic and lipoprotein multiheme cytochromes can interact. The combination of high heme density and arrangement of cofactors results in wire-like electron flow through the complex.
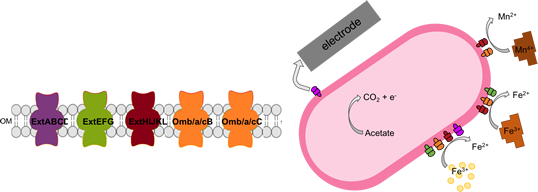
Several putative conduit complexes exist in the genome of G. sulfurreducens, and as described by Jiménez-Otero et al, deleting all possible combinations of these loci reveals how each electron conduit cluster participates in a substrate-dependent pathway. For example, the commonly studied omcB-based conduit is only utilized for electron transfer to metals, while a new cluster named extABCD is the primary conduit for electron transfer to electrodes. Strains expressing only the extABCD conduit cluster grow faster and to higher current densities on electrodes (Jiménez-Otero et al)
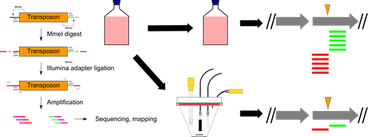
Deep sequencing of mutant libraries: Many of these cytochromes were found using Tn-Seq. If you are working in this model system, these data reveal essential genes and show where insertion mutants could suffer polar effects during engineering (read the Tn-Seq paper or download the raw data here).
Sequencing microbial communities to understand extracellular electron transfer in diverse environments
Genomic sequencing of enrichments from unique environments can bypass the requirement for pure culture isolation, as we seek to understand the evolutionary diversity of extracellular electron transfer. The Soudan Iron Mine (located near Ely, MN) hosts microbial communities a half mile underground that thrive in fractured rock brines up to three times saltier than seawater. We are defining these communities via shotgun metagenomic sequencing supported by the Deep Carbon Observatory's Census of Deep Life. In parallel, we isolate new microorganisms from the deep subsurface that perform extracellular electron transfer under elevated salinity and/or temperature. Through long read/single molecule sequencing we identify otherwise elusive signatures of horizontal gene transfer, gene duplication, and phage infection, all of which appear to play key roles in shuffling the deck of respiratory flexibility in metal-reducing microbes.
New bioreactor designs for reliable microbial electrochemistry
To study electron transfer in electrochemical devices, we need control; the electrode must be the sole electron acceptor, the potential of that surface needs to be known, and biofilms must be captured at precise stages of development. Yet the hand-built reactors used around the world need ports, membranes, and tubing which can introduce variable and large amounts of oxygen. High surface area electrodes, poorly scaled counter electrodes, and internal resistance issues can alter electrode potentials by hundreds of millivolts. Long term operation of reactors, or spikes of donor addition followed starvation, creates biofilms dominated by dead cells.
We machine custom electrochemical reactors to study extracellular electron transfer. The design of these reactors uses small, well mixed, temperature controlled glass vessels, in order to minimize substrate diffusion and internal charge transfer issues, while allowing easy operation of multiple replicates. All electrodes are polished and cleaned before use, reference electrodes are calibrated to an internal standard and cells are inoculated at the same stage of growth. Our current design uses either a 15 or 100 ml reactor fitted with custom-machined tops, and oxygen levels are maintained below 1 ppm. A standard polished 3 cm2 electrode provides plenty of DNA for metagenomics, mRNA for transcriptional analysis, or biofilm for imaging.
PEEK is oxygen impermeable and temperature resistant, allowing use of threaded fittings for all ports, which are further sealed using gasketed compression fittings and stainless steel screws. Reactors built this way can accommodate long-term enrichments, growth of anodic or cathodic strains, be modified to be used as a chemostat, modified to vent gases produced at the counter electrode to the outside, and be be scaled to house multiple electrodes. CAD drawings and instructions for constructing your own are available. 